Nutrition & Cancer
Cancer is the sixth highest cause of death in the world
With 18.1 million new cases and mortality estimated at 9.6 million deaths in 2018 (i.e. 1 in 6 deaths), cancer is considered by the World Health Organization (WHO) to be the sixth highest cause of death in the world [1].
-
-
- Asia alone accounts for 1 out of 2 cancer cases [1]
- Europe accounts for approximately 1 out of 5 cancer cases (23.4 %) [1]
- Similarly the Americas, with approximately 1 out of 5 cancer cases (21 %) around the globe [1]
-
Women are more affected by breast, colorectal, lung and thyroid cancer, while men are more affected by lung, prostate, stomach and liver cancer [1].
Patients with cancer are also undernourished !
An Italian study in 2017 found that 9 % of patients with cancer were undernourished from the first consultation and 43 % were at risk of undernutrition.
According to the European Society for Clinical Nutrition and Metabolism (ESPEN), undernutrition is a frequent feature of patients with cancer regardless of patient age [2]. An Italian study in 2017 found that 9 % of patients with cancer were undernourished from the first consultation and 43 % were at risk of undernutrition [3].
-
-
- On the one hand, undernutrition is a consequence of both the presence of the tumor and the medical and surgical cancer treatments [2]
- At the same time, undernutrition has a negative impact on quality of life as well as tolerance of the treatment [2]
-
ESPEN estimates that 1 to 2 cancer patients out of every 10 die from the consequences of undernutrition rather than from the tumor itself [2].
Nutrition has an important place in the management of cancer
The evidence shows that nutritional issues need to be taken into account from the moment cancer is diagnosed and should be dealt with in parallel with treatment. Despite this, cancer related malnutrition remains widely misunderstood, underestimated and undertreated in clinical practice [2].
How can cancer and its treatment affect nutrition?
Depending on the treatment, cancer sufferers can experience various side effects that affect patient nutrition:[2]
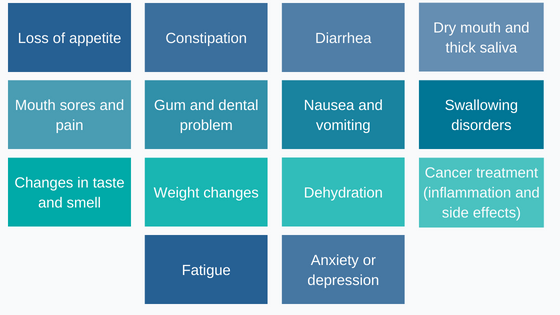
Figure 1 : Patient nutrition can be impacted by many cancer side effects
The patient’s nutrition must above all meet their energy requirements
As with the population at large, the nutrition of a patient with cancer must meet their energy requirements. This means the sum of energy expenditure at rest, during physical activity, and in the small percentage of food-induced thermogenesis.
While energy expenditure at rest is increased, total energy expenditure tends to be reduced, especially in patients with advanced-stage cancer, due to a decrease in daily physical activity [4,5].
ESPEN assumes that total energy expenditure is the same as for a healthy individual and that that requirement is in the region of 25 to 30 kcal/kg body weight/day [2]. For someone who weighs 70 kg that is equivalent to an intake of between 1750 and 2100 kcal.
However, studies show that around 50 % of patients are hypermetabolic, that is to say, have elevated resting energy expenditure [6,7]. It is therefore important to provide more energy to these patients with hypermetabolism. In these cases, the defined energy requirements need to be modified.
Lipids are more easily metabolized by a patient with insulin resistance than carbohydrates
In addition to undernutrition, cancer patients may develop metabolic disorders such as insulin resistance. In these patients, absorption and oxidation of glucose by the muscle cells are altered; however, fat utilization is normal or even increases, which suggests that a higher dietary intake of fats than carbohydrates may be of benefit in ensuring a more efficient energy intake for these insulin resistant patients.
The protein requirements of cancer patients are higher than normal
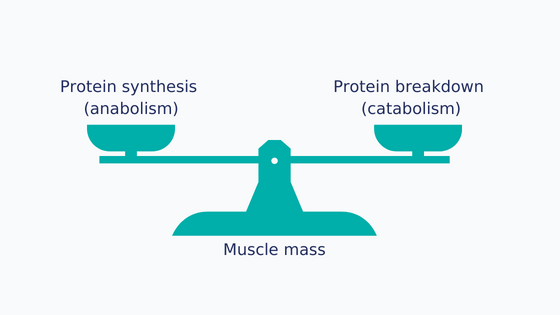
Figure 2 : Balance between anabolism and catabolism
Maintaining muscle mass depends on the balance between the rate of muscle protein synthesis (anabolism) and the rate of breakdown (catabolism) [8–10]. In cancer patients, muscle protein turnover may be disrupted [10,11] for a number of reasons:
-
-
- Reduced protein intake due to inflammation-related anorexia and the adverse effects of cancer treatment contributes to muscle loss [12,13].
- In addition to reduced protein intake, muscle protein synthesis is strongly affected by the level of physical activity [9,14–16], which is often reduced in the case of cancer [17–19], exacerbating still further the problem of catabolism, i.e. protein degradation.
-
This muscle loss in patients is called cachexia: 50 % of all cancer patients suffer from cachexia [20], rising to 60 %-80 % for patients with lung, colorectal, head, stomach or pancreatic cancers [21].
Metabolic studies have shown that high protein intake promotes muscle protein synthesis in cancer patients
Metabolic studies have shown that high protein intake promotes muscle protein synthesis in cancer patients [22].
That is why ESPEN guidelines recommend at least 1 g/kg/day of protein, or even 1.5 g/kg/day when this is possible [2]. For example, for a person who weighs 70 kg that represents between 70 g and 105 g of protein per day. By comparison, the recommended consumption for a healthy adult is 0.8 g/kg/day of protein.
The quality of protein is just as important as the quantity!
The quality of protein is assessed by measuring its composition in terms of essential amino acids as well as its digestibility. The richer a protein is in essential amino acids, the more able the body will be to produce new proteins with it.
In addition to the quality of the protein, the speed of protein absorption is an important factor since the effects on the body vary in accordance with the speed of absorption of the amino acids in the blood.
In general, serum proteins such as whey are classed as so-called “fast” proteins as the passage from the stomach to the intestine is rapid and, during digestion, the amino acids are absorbed in a short time [23]. Finally, it has been demonstrated that the rapid transfer of amino acids to the blood, in particular leucine, promotes increased muscle synthesis [24].
Unlike whey protein, the stomach digests caseins more slowly, releasing it more slowly into blood and for this reason casein reduces protein breakdown and contributes to increased muscle protein synthesis [23,25].
Today, there is data from the literature to show the impact of the quality of proteins on patients with cancer. From these studies, certain results indicate that there are a number of advantages for these patients in consuming milk proteins, especially for patients with cachexia. In these patients, studies show that:
-
-
- The enrichment of a mixture of casein (90 %) and whey protein (10 %) increased protein synthesis in these patients
- Whey protein helped preserve muscle protein during a period of chronic calorie deficit[26]
- Consumption of whey protein during the perioperative period improves physical functional abilities in patients who have had a colorectal resection [27]
- Aside of purely nutritional considerations, patients who received 20 g of whey protein isolate (WPI) for 3 months (vs nutritional advice groups without WPI) were found to exhibit improved body weight, muscle strength and a reduction of chemotherapy toxicity [28]
-
To ensure these nutritional needs are met, dietary advice and oral nutritional supplements are recommended.
This advice includes: [2]
-
-
- First-line dietary advice to encourage the intake of foods and drinks rich in energy and protein that the patient can tolerate
- The treatment of symptoms that have a direct impact on food intake
- Pertinent suggestions for Oral Nutritional Supplements.
-
This nutritional support should preferably be set up before the patient becomes severely malnourished [2].
Supplementation with Oral Nutritional Supplements is advocated when the fortified diet is not effective in achieving nutritional targets:
-
-
- These are indicated when the patient is unable to eat 50 % of normal intake in a week or between 50 % and 75 % of their requirements over 2 days [2].
-
Conclusion
Pronativ® Native Whey Protein and PRONATIV® Micellar Casein are native proteins, extracted directly from milk via a non-denaturing and purely physical process. These products are ideally suited to the management of cancer patients.
Sources :
[1] Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 68, 394–424 (2018).
[2] Muscaritoli, M. et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. Edinb. Scotl. 40, 2898–2913 (2021).
[3] Muscaritoli, M. et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget 8, 79884–79896 (2017).
[4] Gibney, E., Elia, M., Jebb, S. A., Murgatroyd, P. & Jennings, G. Total energy expenditure in patients with small-cell lung cancer: results of a validated study using the bicarbonate-urea method. Metabolism. 46, 1412–1417 (1997).
[5] Moses, A. W. G., Slater, C., Preston, T., Barber, M. D. & Fearon, K. C. H. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br. J. Cancer 90, 996–1002 (2004).
[6] Bosaeus, I., Daneryd, P., Svanberg, E. & Lundholm, K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int. J. Cancer 93, 380–383 (2001).
[7] Cao, D. et al. Resting energy expenditure and body composition in patients with newly detected cancer. Clin. Nutr. Edinb. Scotl. 29, 72–77 (2010).
[8] van Vliet, S., Burd, N. A. & van Loon, L. J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 145, 1981–1991 (2015).
[9] Wolfe, R. R. Regulation of Muscle Protein by Amino Acids. J. Nutr. 132, 3219S-3224S (2002).
[10] Phillips, S. M., Glover, E. I. & Rennie, M. J. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J. Appl. Physiol. 107, 645–654 (2009).
[11] van der Meij, B. S., Deutz, N. E. P., Rodriguez, R. E. R. & Engelen, M. P. K. J. Increased amino acid turnover and myofibrillar protein breakdown in advanced cancer are associated with muscle weakness and impaired physical function. Clin. Nutr. 38, 2399–2407 (2019).
[12] van der Meij, B. S., Teleni, L., Engelen, M. P. K. J. & Deutz, N. E. P. Amino acid kinetics and the response to nutrition in patients with cancer. Int. J. Radiat. Biol. 95, 480–492 (2019).
[13] Dodson, S. et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu. Rev. Med. 62, 265–79 (2011).
[14] Paddon-Jones, D., Short, K. R., Campbell, W. W., Volpi, E. & Wolfe, R. R. Role of dietary protein in the sarcopenia of aging. Am. J. Clin. Nutr. 87, 1562S-1566S (2008).
[15] Phillips, S. M. Nutrient-rich meat proteins in offsetting age-related muscle loss. Meat Sci. 92, 174–178 (2012).
[16] Deutz, N. E. P. et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin. Nutr. 30, 759–768 (2011).
[17] Hasegawa, Y. et al. Protein intake after the initiation of chemotherapy is an independent prognostic factor for overall survival in patients with unresectable pancreatic cancer: A prospective cohort study. Clin. Nutr. 40, 4792–4798 (2021).
[18] Lawson, C., Ferreira, V., Carli, F. & Chevalier, S. Effects of multimodal prehabilitation on muscle size, myosteatosis, and dietary intake of surgical patients with lung cancer — a randomized feasibility study. Appl. Physiol. Nutr. Metab. 46, 1407–1416 (2021).
[19] Smith, W. A., Nolan, V. G., Robison, L. L., Hudson, M. M. & Ness, K. K. Physical activity among cancer survivors and those with no history of cancer— a report from the National Health and Nutrition Examination Survey 2003-2006. Am. J. Transl. Res. 3, 342–350 (2011).
[20] Tisdale, M. J. Mechanisms of cancer cachexia. Physiol. Rev. 89, 381–410 (2009).
[21] Laviano, A. & Meguid, M. M. Nutritional issues in cancer management. Nutr. Burbank Los Angel. Cty. Calif 12, 358–371 (1996).
[22] Baracos, V. E. Skeletal muscle anabolism in patients with advanced cancer. Lancet Oncol. 16, 13–14 (2015).
[23] Boirie, Y. et al. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. U. S. A. 94, 14930–14935 (1997).
[24] Jäger, R. et al. International Society of Sports Nutrition Position Stand: protein and exercise. J. Int. Soc. Sports Nutr. 14, 20 (2017).
[25] Devries, M. C. & Phillips, S. M. Supplemental protein in support of muscle mass and health: advantage whey. J. Food Sci. 80, A8–A15 (2015).
[26] Coker, R. H., Miller, S., Schutzler, S., Deutz, N. & Wolfe, R. R. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr. J. 11, 105 (2012).
[27] Gillis, C. et al. Prehabilitation with Whey Protein Supplementation on Perioperative Functional Exercise Capacity in Patients Undergoing Colorectal Resection for Cancer: A Pilot Double-Blinded Randomized Placebo-Controlled Trial. J. Acad. Nutr. Diet. 116, 802–812 (2016).
[28] Cereda, E. et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 126, 81–88 (2018).